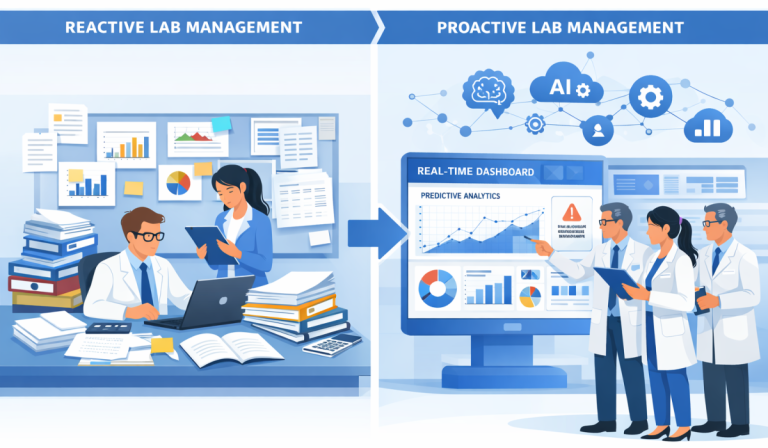
The laboratory industry is changing—fast. Increased regulatory pressure, growing sample volumes, and the demand for real-time data have exposed the limitations of traditional LIMS platforms. While legacy systems like those used by large enterprise labs paved the way, today’s labs need speed, flexibility, and usability—not complexity.
That’s why forward-thinking laboratories are choosing Imara LIMS: a modern Laboratory Information Management System designed for scalability, compliance, and real-world lab efficiency.
The Problem with Traditional LIMS Platforms
Large, established LIMS providers built systems for an earlier era—one dominated by on-premise deployments, rigid workflows, and costly customization. While powerful, these platforms often come with challenges:
-
Lengthy and expensive implementation cycles
-
Heavy reliance on IT and external consultants
-
Complex user interfaces that slow adoption
-
Limited flexibility for evolving workflows
-
High total cost of ownership
For growing laboratories, these limitations directly impact productivity, profitability, and compliance readiness.
What Modern Labs Actually Need from a LIMS
Based on market research and industry benchmarks from global laboratory leaders, today’s labs prioritize:
-
Rapid deployment and faster ROI
-
Configurable workflows without heavy coding
-
Built-in regulatory compliance
-
Intuitive user experience for scientists and QA teams
-
Seamless system and instrument integration
-
Scalable architecture that grows with the lab
Imara LIMS was built with these exact needs in mind.
Imara LIMS: Designed for the Next Generation of Laboratories
Imara LIMS combines enterprise-grade capability with modern usability—without the burden of legacy systems.
1. Faster Implementation, Lower Risk
Unlike traditional LIMS platforms that take months (or years) to deploy, Imara LIMS is designed for rapid configuration and deployment. Labs can go live faster, reduce disruption, and start realizing value sooner.
Result: Faster ROI and minimal operational downtime.
2. Configurable, Not Complicated
Every lab is different—and Imara LIMS respects that. Instead of rigid workflows, Imara LIMS offers configurable processes that adapt to your lab’s needs:
-
Sample lifecycle management
-
Test methods and specifications
-
Approval workflows
-
Reporting and dashboards
All without excessive customization costs.
3. Compliance Built In, Not Bolted On
Regulatory compliance isn’t optional—it’s foundational. Imara LIMS supports compliance with standards such as:
-
ISO 17025
-
GLP and GxP principles
-
Data integrity and audit trail requirements
With automated audit trails, role-based access, SOP control, and validation-ready architecture, Imara LIMS keeps labs inspection-ready at all times.
4. User Experience That Scientists Actually Like
One of the biggest complaints about legacy LIMS platforms? Poor usability.
Imara LIMS delivers:
-
Clean, intuitive interfaces
-
Reduced training time
-
Faster data entry and approvals
-
Higher user adoption across teams
When scientists enjoy using the system, data quality improves naturally.
5. Seamless Integration Across the Lab Ecosystem
Modern labs rely on multiple systems—and Imara LIMS connects them all.
Integration capabilities include:
-
Laboratory instruments (automatic result capture)
-
ERP and MES systems
-
Quality management platforms
-
Analytics and reporting tools
This creates a single source of truth, eliminating silos and manual rework.
How Imara LIMS Competes with Enterprise LIMS Providers
| Feature | Legacy Enterprise LIMS | Imara LIMS |
|---|---|---|
| Implementation Time | Long & complex | Fast & controlled |
| Customization | Costly | Configurable |
| User Experience | Complex | Intuitive |
| Compliance | Add-on heavy | Built-in |
| Scalability | Rigid | Flexible |
| Total Cost | High | Cost-effective |
Imara LIMS delivers enterprise-grade capability—without enterprise-level friction.
Who Is Imara LIMS Built For?
Imara LIMS is ideal for:
-
Food & beverage laboratories
-
Environmental testing labs
-
Contract research organizations (CROs)
-
Pharmaceutical and biotech labs
-
Growing laboratories outgrowing spreadsheets or legacy systems
Whether you’re scaling operations or replacing an outdated LIMS, Imara adapts to your growth.
The Business Impact of Choosing Imara LIMS
Laboratories using modern LIMS platforms experience:
-
Reduced turnaround times
-
Improved audit outcomes
-
Lower operational costs
-
Increased data confidence
-
Better collaboration across teams
Imara LIMS turns quality control from a bottleneck into a competitive advantage.
FAQs: Buying a LIMS
Why should labs replace legacy LIMS systems?
Legacy systems are expensive, rigid, and slow to adapt. Modern labs need flexibility, speed, and usability.
Is Imara LIMS suitable for regulated labs?
Yes. Imara LIMS is designed with compliance, data integrity, and audit readiness at its core.
Can Imara LIMS scale with lab growth?
Absolutely. The platform is built to scale across sites, users, and increasing sample volumes.
Final Takeaway
Choosing a LIMS is no longer just an IT decision—it’s a strategic one. While legacy platforms served their purpose, modern laboratories need a solution that evolves with them.
Imara LIMS delivers the power of enterprise systems with the agility of modern software—making it the smart choice for laboratories ready to grow.
Ready to upgrade your laboratory operations and future-proof your QC processes?
Contact Imara LIMS today:
https://imaralims.com/contact