Why Connected Labs Matter More Than Ever
Let’s be honest—modern QA/QC labs juggle a lot. Instruments generate results, analysts review data, quality teams approve batches, and enterprise systems need timely updates. When these systems don’t talk to each other, labs end up relying on spreadsheets, email attachments, and manual data entry. And that’s where trouble starts.
Disconnected systems slow things down, introduce errors, and create compliance headaches. On the flip side, labs that use Imara LIMS as a connected digital backbone can automate data flow, reduce risk, and keep everyone working from the same, trusted source of truth.
In short, integration isn’t just an IT convenience anymore. It’s a core quality requirement.
Why Integration Is a Quality Requirement, Not a Nice-to-Have
In QA/QC environments, data integrity is everything. Every manual handoff—copying results from an instrument printout to a spreadsheet, then into a batch record—adds risk. One typo can trigger deviations, investigations, or even product delays.
Labs that prioritize integration with Imara LIMS consistently experience:
-
Fewer transcription errors
-
Reduced deviations and rework
-
Faster turnaround times
-
Stronger audit readiness
Regulatory expectations around data integrity, traceability, and audit trails continue to rise. Regulators expect labs to demonstrate not just accurate results, but how those results were generated, reviewed, and approved. Integrated systems make that possible without piling extra work on analysts.
Simply put, if quality data moves manually, quality risk moves with it.
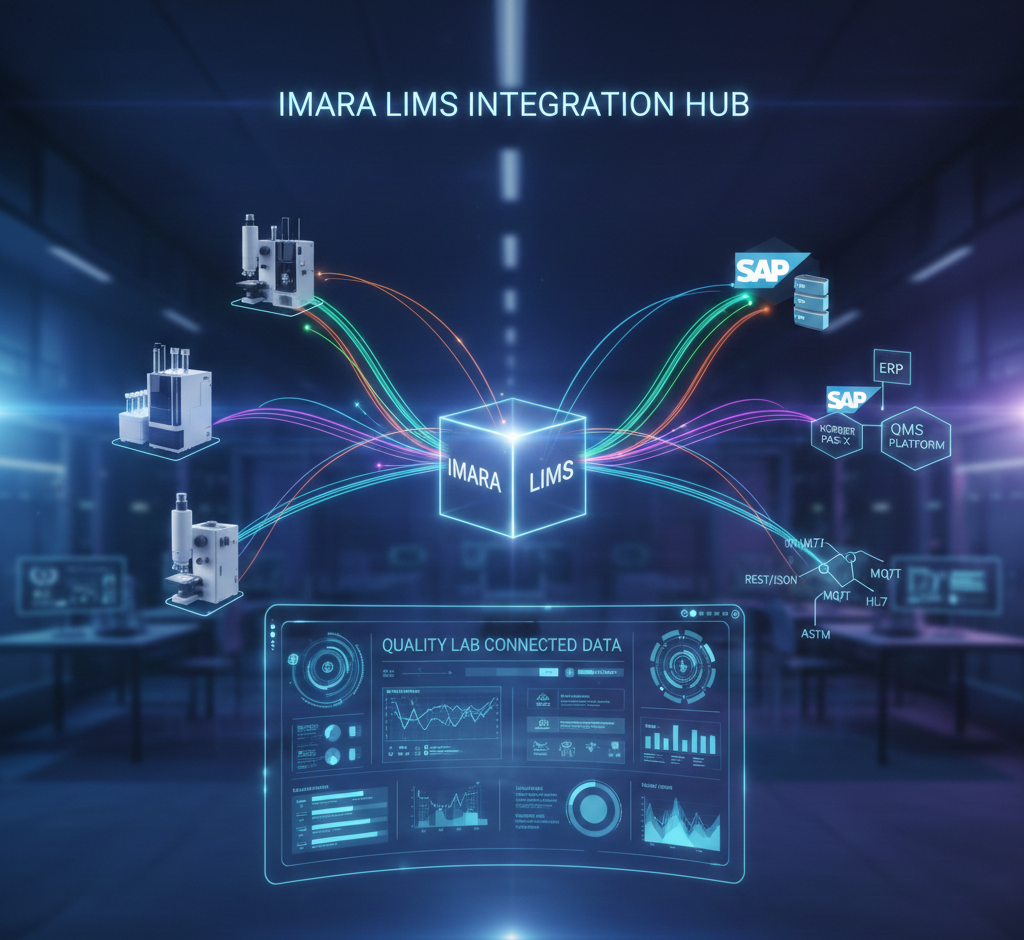
What Imara LIMS Integrates With
Imara LIMS is designed to sit at the center of your laboratory ecosystem, connecting instruments, applications, and enterprise systems through secure and scalable integration patterns.
Instrument Integrations
Imara LIMS can connect directly or indirectly to a wide range of laboratory instruments, including:
-
Balances and scales
-
Chromatography systems (HPLC, GC)
-
Spectrophotometers and plate readers
-
Environmental monitoring devices
Results flow automatically into Imara LIMS along with critical metadata such as method, analyst, timestamp, and instrument ID—creating a complete audit trail without manual intervention.
Chromatography and Scientific Software
Many labs rely on specialized scientific software to manage complex analytical workflows. Imara LIMS integrations support data exchange with commonly used platforms such as:
-
Chromatography data systems (CDS)
-
Plate reader and bioassay software
-
Instrument control and data acquisition tools
Instead of re-entering or reconciling results, analysts can review and approve data directly within Imara LIMS, saving time and reducing errors.
MES, ERP, and Enterprise Systems
Quality data doesn’t stop at the lab door. Manufacturing, supply chain, and quality teams all depend on accurate lab results.
With Imara LIMS integrations, organizations can:
-
Send QC results to MES systems for batch release and production decisions
-
Exchange material, lot, and specification data with ERP systems
-
Align quality events with QMS platforms for deviations, CAPAs, and change control
This ensures production and quality teams are always working with the same, up-to-date information—no guesswork, no delays.
How Imara LIMS Enables Secure and Scalable Integrations
Rather than relying on fragile, one-off scripts, Imara LIMS supports structured integration approaches that scale as your lab grows.
Common Integration Methods
Imara LIMS supports multiple integration styles, including:
-
File-based data exchange
-
API-driven integrations
-
Standards-based messaging
These approaches allow labs to integrate new instruments and systems without disrupting validated workflows or weakening cybersecurity controls.
Data Integrity and Compliance Built In
Every integration with Imara LIMS is designed to preserve:
-
Traceability from result to instrument, method, and analyst
-
Audit trails that meet regulatory expectations
-
Access controls aligned with quality and IT policies
That means automation doesn’t come at the expense of compliance. In fact, it strengthens it.
Real-World Examples of Imara LIMS Integrations
Across industries like pharmaceuticals, biotech, food & beverage, and chemicals, labs use Imara LIMS integrations to solve everyday challenges.
Eliminating Manual Data Entry
By pushing balance and instrument data directly into Imara LIMS, labs eliminate transcription errors and significantly reduce deviations related to data entry.
Aligning QC and Manufacturing
MES–LIMS integration ensures that production and QC teams reference the same material definitions, specifications, and batch data—reducing miscommunication and delays.
Scaling Without Compromising Security
As labs bring more instruments online, Imara LIMS integrations help maintain network security and validation controls while still expanding automation.
Different use cases, same goal: reliable, automated data flow with full traceability.
Operational Gains for QA/QC Teams
Once systems and instruments are integrated with Imara LIMS, the impact shows up quickly in day-to-day operations.
Key Benefits Include
-
Fewer data-entry errors and investigations
-
Faster sample-to-result and sample-to-release timelines
-
Clear traceability from raw data to final decision
-
Less time spent reconciling data during audits
Over time, these benefits compound. As more instruments, methods, and sites are connected, labs gain a consistent and scalable foundation for future initiatives like advanced analytics and AI-driven insights.
Supporting Multi-Site and Global Lab Operations
For organizations operating across multiple sites or regions, consistency is a constant challenge. Imara LIMS integrations help standardize how data is captured, transferred, and reviewed—no matter where the lab is located.
This makes:
-
System upgrades more predictable
-
Validation efforts more manageable
-
Global reporting more reliable
Instead of each site inventing its own approach, integrations follow proven patterns that scale globally.
Where to Go Next With Imara LIMS Integrations
If you’re planning to modernize your quality lab environment, start with a clear map of:
-
Instruments generating critical data
-
Systems that consume or depend on that data
-
Manual steps that introduce risk or delay
From there, prioritize Imara LIMS integrations that eliminate the most manual work or compliance risk first. A phased integration roadmap allows you to deliver value quickly while building toward a fully connected lab ecosystem.
FAQs About Imara LIMS Integrations
What types of labs benefit most from Imara LIMS integrations?
QA/QC labs in regulated industries such as pharmaceuticals, biotech, food & beverage, and chemicals see the greatest benefits due to strict data integrity and compliance requirements.
Can Imara LIMS integrate with existing instruments?
Yes. Imara LIMS supports a wide range of instrument integration methods, allowing labs to connect both new and legacy equipment.
Do integrations increase validation complexity?
When done correctly, integrations actually reduce validation burden by standardizing data flows and eliminating manual processes that are harder to control and document.
Is integration only for large labs?
Not at all. Small and mid-sized labs often see the fastest ROI because even a few automated data flows can eliminate significant manual effort.
Final Thoughts: Building a Connected Quality Lab
Disconnected systems belong to the past. Today’s quality labs need speed, accuracy, and confidence in their data—and that’s exactly what Imara LIMS integrations deliver.
By connecting instruments, lab applications, and enterprise systems into a single, validated ecosystem, Imara LIMS helps labs reduce risk, improve efficiency, and stay inspection-ready as requirements evolve.
Talk to Us
If you’d like to discuss your current lab landscape or explore how Imara LIMS integrations could work in your organization, get in touch with our experts. A short conversation can uncover quick wins and help you start building a more connected, future-ready quality lab.